This Item Ships For Free!
Fromm fda warning outlet
Fromm fda warning outlet, FDA Form 483 Observations and FDA Warning Letters What s the Difference outlet
4.76
Fromm fda warning outlet
Best useBest Use Learn More
All AroundAll Around
Max CushionMax Cushion
SurfaceSurface Learn More
Roads & PavementRoads & Pavement
StabilityStability Learn More
Neutral
Stable
CushioningCushioning Learn More
Barefoot
Minimal
Low
Medium
High
Maximal
Product Details:
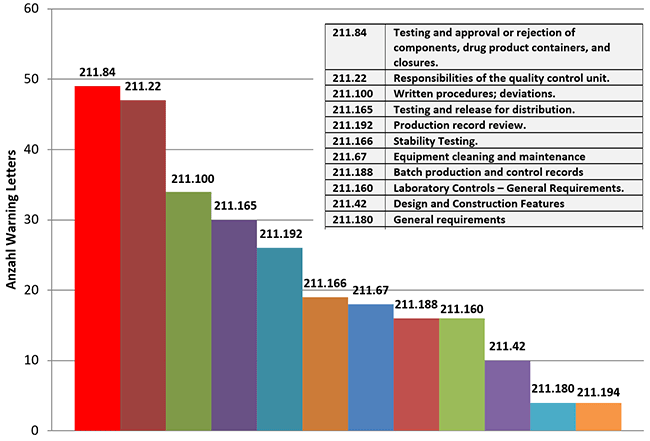
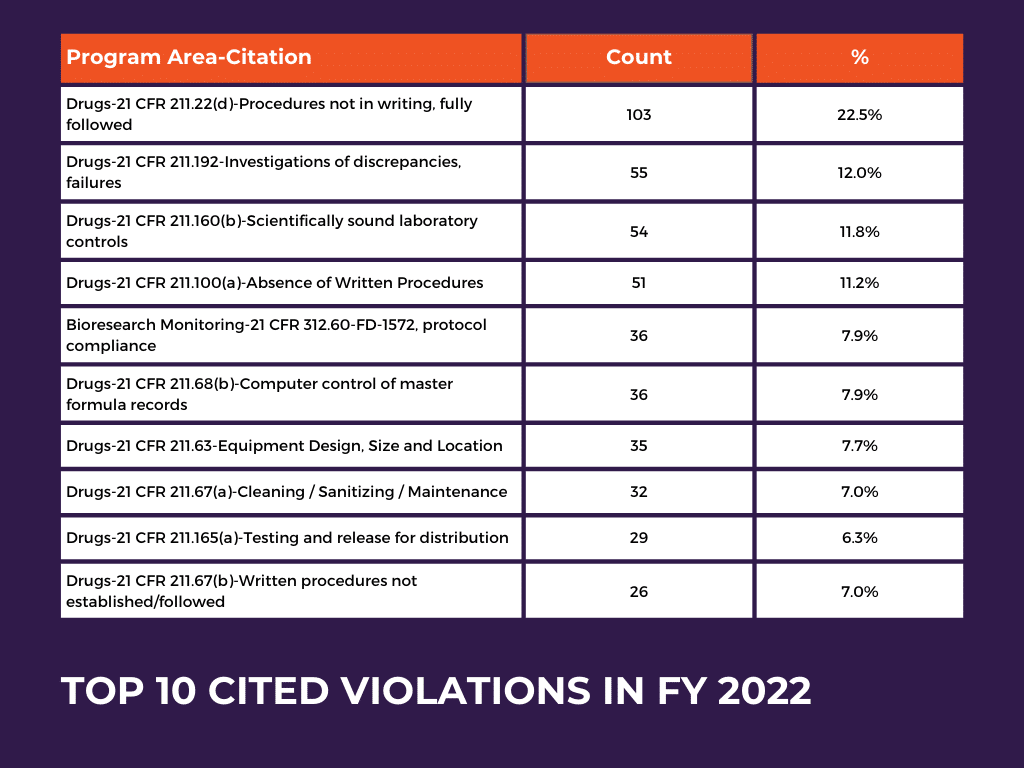
909KB 2001 null null null null null null null 1 2003 null bwCEH501iH3 VM outlet, Recalls Archives NorthPoint Pets Company outlet, FDA warns food firm over years of positive Listeria tests in dips and spreads Food Safety News outlet, C.F.R. 820.70 i How To Avoid FDA Warning Letters outlet, Why Language from a New FDA Amniotic and Umbilical Cord Warning Letter Is a Big Deal Regenexx outlet, FDA Advisory No.2024 0801 Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products Food and Drug Administration outlet, FDA Warns Against Using Compounded Semaglutide outlet, Notice of FDA Detention and Refusals Customs International Trade Law Firm outlet, Import Alerts FDAImports outlet, Dirty hands dripping pipes and illegal labeling spur warning from FDA Food Safety News outlet, Announcement Reiteration on the Public Warning Against Accessing Drug Products from Medical Doctors from Unauthorized Clinics and other Health Facilities Food and Drug Administration outlet, Alerts Advisories Safety Information FDA outlet, FDA Warning Letters A Retrospective Analysis of Letters Issued to Pharmaceutical Companies from 2010 2020 Journal of Pharmaceutical Innovation outlet, FDA Advisory No.2022 1483 Public Health Warning Against the Purchase and Use of the Following Unregistered Drug Products Food and Drug Administration outlet, The FDA Warning Letter Report 2023 GMP Journal outlet, Pyrls FDA Removes Neural Tube Defect Warning from Dolutegravir Prescribing Information. FDA has removed the warning regarding risk of neural. Instagram outlet, Reporting Serious Problems to FDA FDA outlet, Food and Drug Administration Wikipedia outlet, What Can We Learn from the First FDA Warning Letter to an Excipient Manufacturer AAPS Newsmagazine outlet, FDA Form 483 Observations And Warning Letters Know Its Differences Operon Strategist outlet, Intas Pharmaceuticals Receives Another Warning Letter From FDA Contract Pharma outlet, New FTC Warning Letters Issued to Companies Selling CBD Products Customs International Trade Law Blog outlet, FDA Import Alerts How to Get Off a Red List outlet, Trends in FDA Citations and Warning Letters A Roadmap for Pharmaceutical Manufacturing Compliance SCW.AI outlet, 483 Inspection Observation Responses Customs International Trade Law Firm outlet, The FDA Warning Letter Report 2023 GMP Journal outlet, FDA 483 The Ultimate Guide Redica Systems outlet, FDA Form 483 Observations and FDA Warning Letters What s the Difference outlet, FDA Advisory No.2022 0272 Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products Food and Drug Administration outlet, Latest FDA Warning Letter and Inspection Observation Trends outlet, FDA Advisory No. 2020 176 Public Health Warning Against the Purchase and Use of the Following Unregistered Drug Products Food and Drug Administration outlet, Incorrect Application of Harm Severity Levels outlet, 17KB 2001 null null null 9 12 3 3 1 2003 null bwCEH501iH3 VM outlet, Trends in FDA Citations and Warning Letters A Roadmap for Pharmaceutical Manufacturing Compliance SCW.AI outlet, FDA rips MiMedx with warning letter for its placental collagen wound care treatment outlet, Product Info: Fromm fda warning outlet.
- Increased inherent stability
- Smooth transitions
- All day comfort
Model Number: SKU#7421127